Computer chips based on single molecules may remain a work in progress, finds James Mitchell Crow but the technologies developed along the way are being used by chemists to explore their reactions
Squeezing down the size of electronic components to extract more performance from each new generation of gadget is a trend as old as the electronics industry itself. In the early electronics arms race of second world war weapons systems – when cutting-edge electronics buzzed with crystal diodes, vacuum tubes and mercury switches – manufacturers saw gains by moving to miniaturised and then sub-miniaturised vacuum tubes. The smaller their size, the more could be squeezed into the circuitry.
So perhaps it should be unsurprising that the concept of molecular-scale circuitry – as the ultimate expression of electronics miniaturisation – dates right back to the post-war period. In the 1950s, US Air Force researchers were battling with a new generation of electronics based on recently invented silicon transistor technology. Compared to vacuum tubes, transistors were far smaller and ran cooler, enabling the more complex circuit design that was helping USAF hardware to fly higher and faster than ever. However, these flights put the fledgling silicon transistor circuitry through the ultimate stress test, and failures were frequent. Faced with this fragility, USAF engineers were open to alternative electronics technologies.
Single-molecule electronic research is again enjoying a resurgence
In 1956, Massachusetts Institute of Technology physicist Arthur von Hippel laid out a radical, bottom-up approach to electronics design and assembly based on molecular engineering. ‘Instead of taking prefabricated materials and trying to devise engineering applications consistent with their macroscopic properties, one builds materials from their atoms and molecules for the purpose at hand,’ von Hippel argued – three years before Richard Feynman gave his famous ‘Plenty of room at the bottom’ lecture. Inspired by von Hippel’s vision, in 1959 the USAF initiated a US$7 million (around £40 million today) joint research project with Westinghouse to develop molecular electronics.
That project was short-lived. Also in 1959, a new method of silicon integrated circuit manufacture was developed that simultaneously offered the next step in miniaturisation while making the chips cheaper and more reliable. The ensuing exponential progress in silicon chip performance – powered by a doubling of transistor density every two years – was noted in the 1965 publication by Gordon Moore that became known as Moore’s Law.
Although USAF-Westinghouse’s highly ambitious molecular electronics project was canned in 1962, swamped by silicon’s swift progress, the idea of single-molecule circuitry components has regularly resurfaced. Today, silicon transistors are down to single digit nanometre node sizes and rapidly approaching hard physical buffers halting further miniaturisation, so radical alternatives are on the bench once more. Single-molecule electronic research is again enjoying a resurgence.
The increasingly reliable methods for capturing single molecules between pairs of electrodes, meanwhile, are already being rapidly adopted by chemists to address research questions beyond computation.
Plenty of room on an STM tip
Air Force moonshots aside, molecular electronics was arguably born with a 1974 theoretical paper published by Mark Ratner, a theoretical chemist at New York University in the US, and IBM synthetic chemist Ari Aviram. The pair proposed a specific polar organic molecule that could play the functional role of an electronic component called a rectifier.
At the time – aside from the synthetic challenge of making the proposed molecule – multiple technological barriers prevented the concept from being put into practice. There was no way to incorporate an individual molecule into a functioning circuit, let along connect the millions or billions of these molecules that would be needed on a chip.
Scientists modified the STM to make a circuit out of a single molecule
Almost half a century on, major progress has been made toward overcoming that first technological barrier, notes Nadim Darwish, a molecular electronics researcher at Curtin University in Perth, Australia. ‘The scanning tunnelling microscope [STM] was invented to image single molecules, or even single atoms – but as the field advanced, we realised we could not only image molecules but poke them, make electronic contacts with them,’ he says. As an imaging instrument, the microscope tip is held above the surface. ‘A few scientists modified the microscope to make a contact with the individual molecules, creating a circuit out of a single molecule. That initiated single-molecule electronics.’
The typical protocol that emerged is that linear organic molecules with a sulfur-based anchoring group at each end are placed on a gold substrate in the STM. The gold tip is lowered to the surface, then lifted slightly. With luck, one end of the molecule sticks to the tip while the other end remains anchored to the substate, so that the molecule bridges the gap – forming a circuit. ‘You could then measure current passing thought the molecule,’ Darwish says. A related approach can be made using atomic force microscopy, he adds.
But the resulting circuit is fragile. ‘After a fraction of a second, it breaks,’ Darwish says. ‘The nature of the molecule’s contact with gold is still debated – some people call it semi-covalent – but it is not as strong as a proper covalent bond.’ The experiments that can be done on such a short-lived single-molecule circuit are limited. ‘We wanted to make a proper covalent bond to the electrode, so you have a single molecule with a covalent bond at each side.’
Darwish’s strategy was to use silicon rather than gold for the STM tip and substrate. ‘Silicon can form covalent bonds with carbon, oxygen, sulfur – so it provided us with a tool to make the junction survive longer, we could reach several seconds,’ he says. At that timeframe, the team could investigate circuits made from stimuli-responsive molecules to switch the current on and off, akin to a transistor. ‘It was long enough to do some electrochemical gating by switching the molecule between an oxidised and a reduced state, or to shine light and show on–off switchability,’ Darwish says. Related approaches to generate robust molecule–electrode junctions have reached single-molecule circuit stabilities of hours or even days at room temperature, he adds.
Making connections
Xuefeng Guo at the University of Peking in China and his collaborators have taken a similar approach, using carbon nanomaterials in place of gold to form strong covalent bonds to the single molecule bridging the gap. ‘In my lab we have several research directions, but the first is to develop reliable methodologies to make single-molecule junctions,’ Guo says. ‘Our first-generation approach used carbon nanotube electrode-based single-molecule devices, and the second generation used graphene electrode-based single-molecule devices. Now we are developing the third-generation fabrication process based on graphene to form single-molecule circuits with atomic precision, reliable and stable.’
Even with their second-generation junctions, the team recently created a functioning single-molecule version of the critical building block of today’s computer circuitry, the field effect transistor (FET). The FET’s operating principle is that current flow through the transistor, from the source electrode to the drain electrode, can be controlled by a voltage applied to third electrode called the gate electrode, switching the transistor between an on and off state.
For the next step we must combine several components together to have a logic gate
In Guo’s case, the team covalently connected an individual dinuclear ruthenium-diarylene complex between graphene source and drain electrodes, over a metal oxide gate electrode. Current flow through the molecule could be reversibly switched using light-driven ring-opening and -closing reactions. In ring-open form, however, current could also be controlled using voltage applied to the gate. The device therefore operated as a solid-state single-molecular FET, stable at room temperature. ‘It is quite promising,’ Guo says. ‘If we can solve the problem of integrating these devices together, the future is coming soon.’
As Wenjing Hong of Xiamen University in China can attest, however, that’s easier said than done. To build a single-molecule mimic of conventional computer electronics, wiring more than one molecular component into a circuit will be a critical step because a functioning logic circuit requires multiple components – for example, a transistor plus a diode. ‘When I completed my PhD in Switzerland in 2013, I wrote in my thesis, “For the next step we must combine several components together to have a logic gate,”’ Hong recalls. ‘But until now, nobody has really achieved it. We cannot build the basic gate circuit for any kind of integrated circuit. This becomes the biggest bottleneck.’
In 2019, Hong and his collaborators published their own molecular transistor, built from a thiophene-based molecule anchored between gold electrodes. Despite considerable effort, however, the team has been unable to string two single molecules together in a circuit. ‘We spent a huge effort – I put almost 10 PhD and master’s students on this project, with the idea to connect two transistors together. Not from one to 1 million transistors, just from one to two – but we didn’t succeed even once,’ he says. ‘We have to find another way.’
Enter the chemists
As a way forward, Hong is looking to chemistry to work his way around the problem. Rather than organic molecules that directly replicate the function of conventional electronic components such as transistors and diodes, the concept is to design multifunctional molecules that can operate as a logic gate in their own right – thereby removing the need to wire up multiple molecules into each logic circuit.
The increasing focus on the chemistry reflects a wider trend, Darwish notes. ‘For a long time, the field was dominated by physicists, focused on the electrical engineering,’ he says. ‘More recently, many chemists have become involved.’
The interest is two-fold, Darwish says. ‘As well as using the molecules to make electronics, we can use the electronic setup to study chemical reactions. The maturity of the technique has now reached the point that many chemists have access to it. In my opinion, the chemical uses of these techniques will have real world outcome before molecular electronics applications,’ he adds.
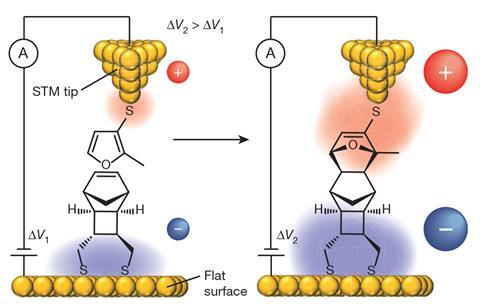
One concept is electrostatic catalysis: the use of a strong electric fields to selectively drive the movement of electrons associated with a particular bond-making or -breaking event, toward a desired product. For example, Darwish and his collaborators have explored the STM tool to study, at the single-molecule level, the electrostatic catalysis effect on the Diels–Alder reaction. For practical synthetic applications, one possibility could be to use microfluidics to flow reactants between two closely spaced electrodes, Darwish says.
Novel applications of STM single-molecule junctions are also being explored by Guo and his colleagues. ‘On the basis of our third-generation device fabrication platform, we are introducing some preliminary research in combination with biology, chemistry, materials, physics,’ Guo says. ‘For example, we are using the single-molecule devices to visualise the dynamic process of organic chemical reactions, so you can reveal the intrinsic mechanism and develop new chemistry.’
Guo recently used the tool to resolve longstanding debate over the mechanism of the classical Suzuki–Miyaura cross-coupling, a mainstay reaction of organic synthesis. ‘The temporal resolution of the STM technique is so high, tens of gigahertz bandwidth, that you can repeat the measurements at nano- to pico-second resolution to follow the steps of the reaction,’ Darwish says. By trapping the Suzuki–Miyaura’s N-heterocyclic carbene–palladium catalyst between a pair of graphene electrodes, each step in the catalytic cycle could be observed, and the precise mechanism of the transmetallation step elucidated. ‘This is not possible by any other technique,’ Darwish says. ‘Many chemists are very interested in this aspect of the technology.’ Other classic reactions recently studied by the technique include nucleophilic substitutions.
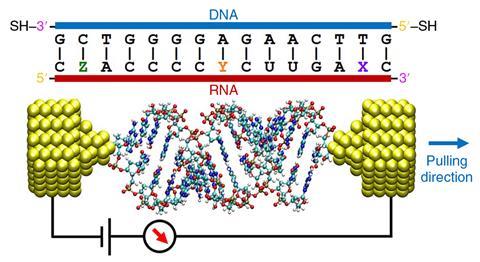
Others are using the technology as an electronic sensor, for example to extract sequence information from short strands of RNA. ‘Another area of research is to develop the field of single-molecule biophysics for medical applications,’ says Guo. ‘You can reach the ultimate goal of single-molecule detection, for accurate point of care diagnosis.’ Using a technique called dielectrophoretic trapping, polar molecules in the sample, such as DNA or proteins, can be drawn into the electrode gap by the application of an electric field.
The tool also allows the fundamental properties of individual molecules to be probed. ‘I like the fundamental information about electronic structure that one gets from single-molecule transport studies,’ says Luis Campos, a chemist at Columbia University in New York City, US. ‘It helps us understand macromolecular properties from their building blocks.’
Campos recently co-led a study to generate stable oligophenylene-bridged bis(triarylamines) with mono- and di-radical cation character that, when individually anchored between two gold electrodes, performed as highly conductive molecular wires. Thanks to its radical nature, conductance through the wire increases with increasing length of the molecule, the team showed.
Extracting information from single molecules and implementing those concepts in chemistry can be still quite the challenge, Campos notes – but extremely valuable. ‘Getting information from photochemical processes in single molecules is still quite difficult, and the same for spin processes imparted by both open shell systems and excited states. But this is certainly coming, from the chemistry point of view,’ he says.
Hello world
Chemists might be enjoying the tools that have been developed in the quest for the molecular nanocomputer – but chemistry can, in turn, also contribute to the original goal of single-molecule electronic circuitry.
Hong and his team were using single-molecule experiments to explore the electrostatic catalysis effect on organic reactions when inspiration struck. ‘We started to wonder if we can use this effect to manipulate a single-molecule logic gate,’ he says. Rather than connect more than one molecule into a circuit to achieve logic function, could a multifunctional single molecule – one that could be flipped between two states using the electric field – achieve the same effect, eliminating the need to wire multiple molecules into the circuit?
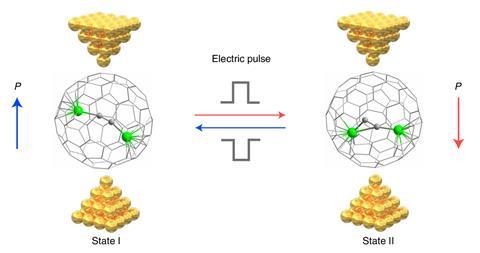
In July 2022, the team published their latest step forward, a room temperature stable metallofullerene-based single-molecule memristor device. The C88 fullerene cage contains a [Sc2C2] group whose orientation can be flipped between two stable states using voltage pulses. As the current flow through the molecule differs in each state, the molecule could perform as a logic gate, and also be used for data storage.
‘Originally this work was not for electronics at all, it was to study chemical reactions, but we found this effect can be used to rotate the dipole of the metal cluster in these fullerenes,’ Hong says. ‘Now we have the molecular logic gate, in principle we can design a chip with molecular device which can have some basic logical function. Even it if it is just like “Hello world!”, “Hello molecular computer!”’
The target now is to integrate 100 or even 1000 fullerene logic gates in a chip, Hong says. ‘We are trying hard to achieve something in time to dedicate to the 50th birthday of molecular electronics, 50 years since the very famous 1974 paper by Ratner and Aviram,’ he says.
‘If we can make a chip in two years as we expect, this technique is still very far from industry,’ Hong adds. ‘But even if it is very slow and very stupid, even if it is not economic or efficient, no better than silicon – if it demonstrates very basic function, it is still a big step forward for molecular electronics and for chemists to really think about the future.’
James Mitchell Crow is a science writer based in Melbourne, Australia







1 Reader's comment