This activity is intended for pulmonologists, transplant surgeons, hematologists, and internal medicine specialists.
The goal of this activity is for learners to be better able to enable an improved understanding of the high stakes of bronchiolitis obliterans syndrome (BOS) for lung transplant recipients and the crucial importance of identifying these patients as soon as possible.
Upon completion of this activity, participants will:
Medscape, LLC requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated. Others involved in the planning of this activity have no relevant financial relationships.
Disclosures for additional planners can be found here.
Medscape, LLC designates this enduring material for a maximum of 0.25
AMA PRA Category 1 Credit(s)™
. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.25 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Aggregate participant data will be shared with commercial supporters of this activity.
For questions regarding the content of this activity, contact the accredited provider for this CME/CE activity noted above. For technical assistance, contact [email protected]
There are no fees for participating in or receiving credit for this online educational activity. For information on applicability
and acceptance of continuing education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those
credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the
activity online during the valid credit period that is noted on the title page. To receive AMA PRA Category 1 Credit™, you must receive a minimum score of 70% on the post-test.
Follow these steps to earn CME/CE credit*:
You may now view or print the certificate from your CME/CE Tracker. You may print the certificate, but you cannot alter it.
Credits will be tallied in your CME/CE Tracker and archived for 6 years; at any point within this time period, you can print
out the tally as well as the certificates from the CME/CE Tracker.
*The credit that you receive is based on your user profile.
CME / ABIM MOC Released: 12/28/2022
Valid for credit through: 12/28/2023, 11:59 PM EST
processing....
In lung transplantation recipients, bronchiolitis obliterans syndrome (BOS) is one of the phenotypes of chronic lung allograft dysfunction (CLAD) and remains the major limitation of long-term survival. BOS affects up to 50% of lung transplant patients within 5 years post-transplantation. To date, there is no cure, so management of BOS is carried out on a case-by-case basis and relies on a thorough understanding of its pathology. We recently spoke with Dr Deborah Levine, clinical professor at Stanford University, about the pathogenesis, outcomes, and recent advancements in the search of a treatment for BOS.
Medscape: What is BOS and how do you recognize it in lung transplant recipients?
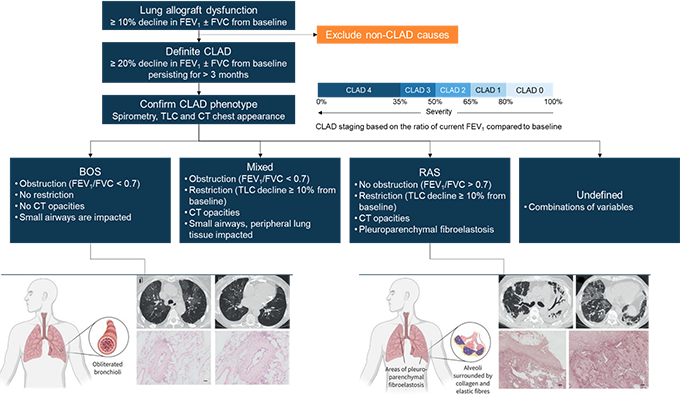
Deborah Jo Levine, MD: BOS after lung transplantation is a syndrome characterized by fibrosis and obliteration of the airway, with a resulting decline in the forced expiratory volume in 1 s (FEV1). BOS occurs in most patients within 5 years post-transplantation. The International Society for Heart and Lung Transplantation (ISHLT) defines BOS as a persistent decline in pulmonary function. That means a decrease of ≥ 20% from the reference value in FEV1 lasting for at least 3 months, after excluding other potential causes. We calculate the reference FEV1 value as the average of the best 2 post-transplant FEV1 values, measured at an interval of at least 3 weeks. The fall in FEV1 can also be accompanied by a change in the forced vital capacity (FVC), although this might not always be the case.[1] BOS presents clinically as an obstructive dysfunction which affects the small airways; however, the peripheral lung tissue is less impacted (Figure 1). Patients are therefore showing stable or increasing total lung capacity (TLC) and declining FEV1/FVC (< 0.7).[1] Computed tomography (CT) and hematoxylin and eosin staining can also support diagnosis of BOS. At baseline, a CT scan may not show significant abnormality, but as BOS evolves, air trapping and bronchiectasis can be detected, while hematoxylin and eosin staining will show subepithelial fibrosis restricting the airway lumen (Figure 1).[2] There is no typical course for BOS, and lung function after BOS onset is highly variable. Some patients may experience significant decline in lung function early after the onset of BOS, while for others lung function can remain stable for long periods, despite being reduced.
Figure 1. CLAD Diagnosis, Phenotype Confirmation, and Staging in Lung Transplant Patients
An overview of BOS and restrictive allograft syndrome (RAS) characteristics is provided, with CT and staining findings that can help differentiate between the 2 phenotypes. BOS air trapping and bronchiectasis revealed by CT; hematoxylin and eosin staining shows typical bronchiolitis obliterans lesion, characterized by concentric subepithelial fibrosis narrowing the airway lumen. RAS: bilateral opacification of lung lobes, volume loss, traction bronchiectasis areas of ground-glass opacification, and interlobular septal thickening revealed by CT; Elastica van Gieson stain shows prominent pleural fibrosis and subpleural fibroelastosis, consistent with pleuroparenchymal fibroelastosis.[1,2] Figure partially reproduced from Bos et al.[2]
Medscape: How is BOS severity staged?
Dr Deborah Levine, MD: BOS severity is determined by the magnitude of the decline in FEV1. In the past, severe BOS was defined as a decline in FEV1 ≤ 50% of the reference value.[3] However, the ISHLT guidelines have been updated and all CLAD clinical phenotypes are now included in the severity staging, and are graded from CLAD0 to CLAD4 (Figure 1). Grade 4 CLAD -- and therefore BOS -- is now defined as FEV1 ≤ 35% of the reference FEV1 value.[1]
Medscape: How can you distinguish BOS from the other CLAD phenotypes?
Dr Levine: BOS is by far the most common manifestation of CLAD, in 50% to 70% of cases.[4-6] Another phenotype, RAS, has been reported to occur in up to 30% of patients with CLAD,[1] although recent studies have shown a lower percentage of around 9%.[5] There are also mixed or undefined phenotypes (Figure 1). In contrast to BOS, RAS shows restrictive physiology without evidence of obstruction, and CT will show persistent opacities due to pulmonary and/or pleural fibrosis.[1,7] It is important to know that BOS can transition to RAS, or rather to a mixed phenotype of CLAD with both obstructive and restrictive physiology. More seldom, RAS can also evolve to mixed CLAD.[1,4] And then there are also undefined phenotypes, when CLAD is confirmed but difficult to classify, although these are yet to be better characterized.[1] All these definitions require further validations and will most likely evolve in the future, but so far refining CLAD -- and by extension BOS -- definitions has allowed considerable improvements in the management of this condition.
Medscape: What are the tools used to diagnose and monitor BOS?
Dr Levine: BOS is diagnosed based on the ISHLT criteria, using pulmonary function testing and imaging techniques. These are also the main tools used to assess response to treatment during BOS. Once a patient who has received a lung transplant shows a decline in FEV1 of ≥ 10%, an evaluation is done to look for causes of this drop. The pulmonary function tests are monitored after that so that BOS can be detected as early as possible. Lung function -- by standard spirometry -- is evaluated at every clinic visit and at least 3 to 4 months apart.[1] Home spirometry can also be used if there is a need to reduce clinic visits, as was the case during the COVID-19 pandemic.[4] TLC is measured by plethysmography at 3 and 6 months post-transplantation and at all annual visits, but also when a decline in FEV1 of ≥ 10% is observed.[1] Imaging techniques (mainly CT) are complementary in the diagnosis/monitoring of BOS, but they be very useful especially after single lung transplantation. In this case, differentiating between CLAD phenotypes is more difficult, due to the contribution of the native lung to pulmonary function tests.[8]
Medscape: What is known about the cause of BOS?
Dr Levine: Currently, there is not one specific known cause for BOS. Several mechanisms have been proposed for the injury and inflammation of the small airways, which can lead to damage to the epithelium or other tissues. I can mention here alloimmune responses -- either acute cellular rejection (ACR) or antibody-mediated rejection -- or autoimmune processes involving exposure of airway-specific autoantigens such as collagen type V and Ka-1 tubulin. External alloantigen-independent stimuli targeting airways (such as pathogens, aspiration, or pollution), or ischemia and early post-transplant events leading to microvascular damage around small airways have also been proposed.[7] Evidence for these potential mechanisms relies on the risk factors identified for BOS: non-adherence to or suboptimal immunosuppressive regimens, ACR episodes, donor-specific antibodies, lymphocytic bronchiolitis, community-acquired respiratory viral infection, air pollution, Pseudomonas aeruginosa or Aspergillus fumigatus colonization, and gastroesophageal reflux disease (GERD).[4]
Medscape: What does survival look like for lung transplant recipients with BOS?
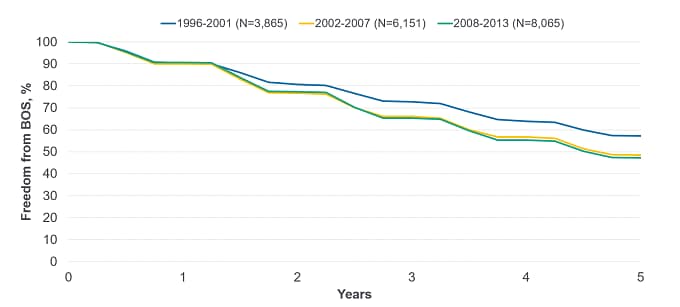
Dr Levine: It is important to realize that BOS affects up to 50% of patients receiving lung transplants within 5 years post-transplantation (Figure 2).[4] Most patients (almost 90%) develop BOS or die within 10 years after transplantation, and almost all survivors have BOS by this time.[9] In patients receiving lung transplants between 1994 and 2011, median BOS-free survival was estimated at 3.16 years and 3.58 years for single and bilateral lung transplant recipients.[9] However, in a more recent clinical trial, median survival was estimated at 95 months (around 8 years) for BOS-related death vs 118 months (roughly 10 years) for non-BOS related death.[10]
Figure 2. Freedom From BOS Conditional on Survival to Discharge by Era for Lung Transplant Recipients (ISHLT Registry Report, 2021)
Freedom from BOS at 5 years after transplantation for transplants reported to ISHLT between 1996 and 2013 was 57% for transplants occurring in the 1996 to 2001 period, and decreased to 49% and 47% for the 2002 to 2007 and 2008 to 2013 time periods, respectively.[11] Figure adapted from the International Thoracic Organ Transplant (TTX) Registry Data Slides 2021, Adult Lung Transplantation Focus Theme (https://ishltregistries.org/downloadables/slides/2021/Adult_Lung_Transplantation_Focus_Theme.pptx)
Medscape: What are some predictors for worse survival in patients with BOS?
Dr Levine: Early-onset BOS is associated with worse survival than late-onset BOS.[12,13] High-grade BOS onset (grade of 2 or 3) after bilateral lung transplantation is also associated with poor prognosis, regardless of timing.[12] Transition from BOS to RAS also leads to decreased survival compared to patients with persistent BOS.[14] The decline in FEV1 has been used as surrogate of mortality for patients with CLAD, based on the assumption that irreversible and progressing loss of lung function will expedite death. However, empirical confirmation was only recently reported by Kneidinger et al; in a clinical trial following up lung transplant recipients for 10 years, each 1% decline in FEV1 was found to predict a 3.4% increase in mortality risk.[10] A ≥ 10% decline in FEV1 and FVC occurring within 6 months of CLAD was also proposed as a surrogate measure of graft survival.[6]
Medscape: What about the quality of life (QOL) of patients living with BOS?
Dr Levine: Patients with BOS typically experience dyspnea, fatigue, and persistent and progressive cough, although they can be asymptomatic during the early stages of the syndrome. There are very few studies assessing the impact of BOS on the patients' QOL after lung transplantation.[15-18] One such study reported a significantly negative impact of BOS on the patients' well-being and QOL.[16] In another, patients with BOS reported a more pronounced impact on energy and physical mobility, as well as more depressive symptoms and anxiety compared to patients without BOS.[17] Nevertheless, in all studies, at least temporary improvement in the QOL was found post-transplantation, even in patients with BOS.
Medscape: What are the current treatment approaches for BOS?
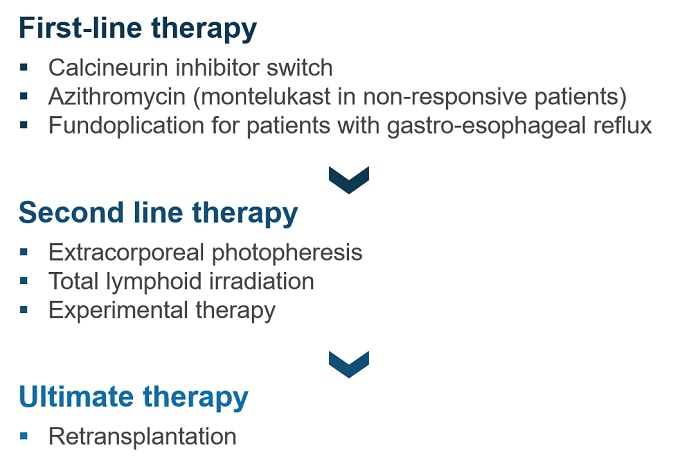
Dr Levine: There are no current established therapies or specifically approved drugs. Management of BOS is considered on a case-by-case basis, depending on the grade at onset or the presence of underlying and/or precipitating conditions (Figure 3). Optimizing immunosuppressive therapies may mitigate progression to higher grades for BOS. The ISHLT recommends a switch from cyclosporine to tacrolimus (which can help stabilize lung function) in patients receiving cyclosporine therapy.[1,3] A course of azithromycin for ≥ 8 weeks is recommended even before onset of CLAD/BOS,[1,3] as it can improve FEV1 and is generally well tolerated.[19] In patients not responding to azithromycin, montelukast can be used, although the evidence is less robust in its case.[20] Fundoplication is recommended for patients with confirmed GERD.[1,3] Second-line therapeutic options may include extracorporeal photopheresis or total lymphoid irradiation.[1,4] There is some evidence that both these therapies slow down the decline in FEV1; however, they also have clear disadvantages. Extracorporeal photopheresis is not generally available and has a high cost. Not all patients can complete treatment with total lymphoid irradiation due to bone marrow suppression or infection.[4] For advanced BOS, transplantation may be the only therapeutic option available if patients are eligible.[1]
Figure 3. ISHLT Recommendations for the Treatment of BOS[1]
Medscape: What are the most promising experimental therapies for BOS?
Dr Levine: The agent in the most advanced stage of clinical development is the aerosolized liposomal cyclosporine (liposomal cyclosporine A for inhalation[L-CsA-i]), administered directly to the allograft. Its efficacy is investigated in the ongoing BOSTON clinical trials; 2 of these trials (BOSTON-1/NCT03657342, BOSTON-2/ NCT03656926, and their extension BOSTON-3/NCT03657342) are phase 3 clinical studies that enroll patients with BOS after single- or double-lung transplantation. L-CsA-i was shown to be well tolerated in lung transplant recipients[21] and has already received orphan drug designation for the treatment of BOS from the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), and Fast Track designation from FDA. The antifibrotic drug nintedanib has also reached the phase 3 clinical trial stage for the treatment of BOS in lung transplant recipients and is currently being investigated in the INFINITx-BOS trial conducted in France (NCT03283007). Results of these trials are awaited in the next few years. B-cell-directed therapies are also considered, but so far the evidence for their use in the treatment of BOS is scarce.[4]