A quick search for "antibacterial soap" on many online retailers' websites will bring up dozens of products, some of which appear to contain an active ingredient that has been banned from the market.
The website of retail pharmacy giant Walgreens, for example, lists Dial Complete antibacterial soap with the active ingredient triclosan, a chemical the US Food and Drug Administration (FDA) banned along with others in 2017. The agency cited a lack of evidence that the ingredients were more effective than plain soap and water and that they were safe for long-term daily use.
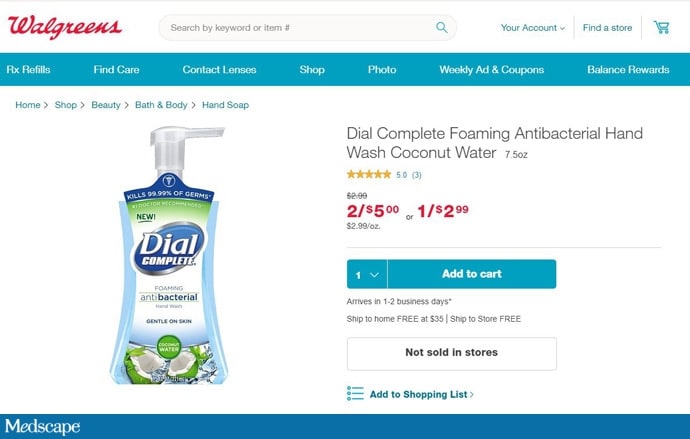
A Dial Complete soap product page on Walgreens' website lists, as of February 4, 2020, an ingredient that was banned by the FDA.
Yet banned substances such as the triclosan in this Dial soap still commonly appear on online product descriptions, researchers found after searching the National Drug Code Directory and the websites of major online retailers, including Amazon, Walmart, and Target. The health effects of antibacterial ingredients "are very poorly defined," said Chandler Rundle, MD, first author of the study, which was published in Dermatitis. Rundle is with the Department of Dermatology at the University of Colorado School of Medicine in Aurora.
The label on the back of the Dial soap bottle sold on Walgreens.com states that it "[k]ills more bacteria than ordinary liquid hand soap." The website displays a close-up graphic of a hand that has been washed with Dial soap and that has fewer bacteria than a hand washed with "Others." The graphic includes a dramatization disclaimer.
When asked about the product, a Walgreens corporate relations spokesperson checked the soap's ingredients list they had on file from Dial's parent company, Henkel North American Consumer Goods.
"I did not see that particular ingredient," the representative said. Their ingredients list reflected a version of the soap that was updated after the ban. That label differs from Walgreens.com's product information. The updated, ban-compliant version of the soap contains an alternative antibacterial compound, benzalkonium chloride. The spokesperson wasn't sure of the source of the incorrect information on the website. Dial did not respond to a request for comment.

The ingredients list for Dial Complete soap on Walgreens.com shows FDA-banned triclosan as the active ingredient.
The 2017 FDA ban restricted the marketing of triclosan and triclocarban along with 17 other ingredients in consumer antibacterial soaps because manufacturers did not provide sufficient data to demonstrate that the ingredients were safe and effective, according to the FDA's announcement. Independent research also showed that some ingredients worked no better than traditional soap and could create antibacterial-resistant microbes. Regular hand soap "still kills bacteria," Rundle said. "The inclusion of an antibacterial substance does not make it better."
Retailers (such as Walgreens) aren't required to update their products' online ingredient lists, which can pose a challenge for people who suffer from skin allergies, said Rundle. People at risk of having a reaction must read labels to verify the ingredients that are included.
Consumer antibacterial soap products that contain the banned compounds have largely been replaced with stand-ins, such as benzalkonium chloride and chloroxylenol, according to Rundle's study. He and other researchers are trying to determine whether those compounds have the same shortcomings. "We're talking 10 to 20 years down the line, and we're worried about things like antibacterial resistance and systemic effects," Rundle said.
The FDA has considered a ban on benzalkonium chloride and additional antibacterial ingredients, but in 2016, it granted ban deferrals, pending more research. The agency exchanged letters with the American Cleaning Institute (ACI), a trade association that represents companies, including the Henkel Corporation. The FDA required that its companies fund research to show that the new antibacterial ingredients are safe and effective. The FDA granted subsequent annual extensions in 2017, 2018, and, most recently, in August 2019 to allow continued research into whether several ingredients are effective in soaps. In its most recent letter to the ACI, the FDA gave a checklist of research tasks to be submitted by July 2020.
The letter from August 2019 stated that the ACI, in its March 2019 progress report, failed to address milestones in studies of healthcare personnel handwashing for two of the substances. It also referenced the ACI's lack of funding for the studies and reminded the organization that further deferrals would not be granted unless the ACI can show ongoing progress.
The ACI plans to meet with the FDA to have an in-depth discussion, Brian Sansoni, a spokesperson for the ACI, told Medscape Medical News. The ACI plans to give the FDA data that show the effectiveness of these ingredients over the course of several years, "due to the complexity of what FDA is asking for," Sansoni said. "We're working as diligently as possible to meet FDA requests."
Leto Sapunar is a freelance journalist and graduate student in New York University's Science Health and Environmental Reporting Program. He studied physics at Oregon State University and writes primarily about science and health.
Dermatitis. 2019;30:352-357. Abstract
Follow Medscape on Facebook, Twitter, Instagram, and YouTube. Here's how to send Medscape a story tip.
Medscape Medical News © 2020
Cite this: 'Antibacterial' Soap Labels Still List Banned Ingredients - Medscape - Feb 04, 2020.







Comments